The Restore EF Study demonstrates the use of contemporary best practices, including attempting a more complete revascularization with Impella-supported high-risk PCI, is associated with significant improvement of left ventricular ejection fraction (LVEF), heart failure symptoms, and anginal symptoms at follow up. The interim analysis was presented today by Mitul Patel, MD, an interventional cardiologist at UC San Diego Health, at TCT Connect, the 32nd annual scientific symposium of the Cardiovascular Research Foundation.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201014005286/en/
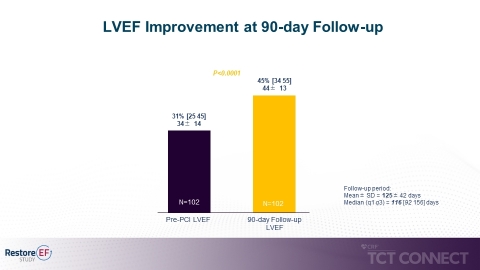
Figure 1 (Graphic: Business Wire)
The ongoing, multi-center, prospective, single-arm study enrolled 193 consecutive qualified patients who underwent a Protected PCI procedure with Impella between September 2019 and September 2020 at 19 hospitals in the United States, representing a variety of hospital settings including rural, urban, community and academic centers. The interim analysis showed:
- Significant median LVEF improvement from baseline to 90-day follow up (31% to 45% p<0.0001). LVEF improvement at 90 days is the study’s primary endpoint. (see figure 1)
- Significant reduction of heart failure symptoms with 80% reduction in New York Heart Association (NYHA) classification III/IV at follow up (54% to 11% p<0.001). (see figure 2)
- Significant reduction of anginal symptoms with 99% reduction in Canadian Cardiovascular Society (CCS) classification III/IV at follow up (70% to 1% p<0.0001). (see figure 3)
“Restore EF demonstrates Impella-supported PCI patients have shown a significant LVEF improvement at 90 days. The study also found a significant improvement in heart failure and anginal symptoms assessed with NYHA and CCS functional classifications,” said Dr. Patel. “Taken together, this data validates best practices for treating high-risk PCI patients, including the use of Impella to achieve a complete revascularization in a single setting.”
“High-risk PCI patients often pose a revascularization challenge due to patient comorbidities, poor LV function, and adverse hemodynamics, which drive worse outcomes. This research demonstrates the rationale for using Impella support during high-risk PCI to maintain coronary perfusion and support hemodynamics during periods of myocardial ischemia during long or repeated balloon inflations or atherectomy runs. This allows providers to achieve complete functional revascularization and the best possible outcomes for our patients,” said Jason Wollmuth, MD, an interventional cardiologist at Providence Health and Vascular Institute and a co-principal investigator of the Restore EF Study.
Restore EF is part of a growing body of evidence demonstrating Protected PCI with Impella is associated with improvements in LVEF and heart failure symptoms. That research includes:
- Burzotta et al., which found Protected PCI with Impella is associated with LVEF improvement in complex high-risk patients at 90 days (27% vs. 33%, p<0.001). The authors also found more complete revascularization is associated with increased LVEF and survival.
- PROTECT II Randomized Controlled Trial, which found Protected PCI with Impella led to a 58% improvement in NYHA class III and IV heart failure symptoms at 90 days (p<0.001). The trial also found, during follow up after Protected PCI with Impella, patients had a 22% improvement in LVEF (p<0.001).
- Maini et al., which found a 17% improvement in LVEF at follow up after a Protected PCI with Impella (p<0.0001).
Findings from Restore EF will be used to inform the study protocol for the upcoming PROTECT IV Randomized Controlled Trial. PROTECT IV will be a prospective, two-arm trial that will compare complete revascularization PCI with Impella to complete revascularization PCI without any planned hemodynamic support. PROTECT IV is part of the Impella clinical evidence pathway to a Class I clinical guideline recommendation for high-risk PCI.
The Restore EF study is sponsored by Abiomed and reflects the company’s commitment to investing in clinical research to improve patient outcomes.
To share best practices in high-risk PCI, Abiomed is hosting a symposium at TCT Connect on Saturday, October 17, at 2:00 p.m. EDT, titledProtected PCI in COVID-19 Era: The Rise in Importance of Complete Revascularization. The symposium is chaired by Cindy Grines, MD, chief scientific officer of Northside Hospital Cardiovascular Institute in Atlanta and president of the Society for Cardiovascular Angiography and Interventions (SCAI). It will feature best practices for using percutaneous mechanical circulatory support to enable complete revascularization in high-risk patients.
ABOUT IMPELLA HEART PUMPS
The Impella 2.5® and Impella CP® devices are U.S. FDA PMA approved to treat certain advanced heart failure patients undergoing elective and urgent percutaneous coronary interventions (PCI) such as stenting or balloon angioplasty, to reopen blocked coronary arteries. The Impella 2.5, Impella CP, Impella CP with SmartAssist®, Impella 5.0®, Impella LD®, and Impella 5.5® with SmartAssist® are U.S. FDA approved heart pumps used to treat heart attack or cardiomyopathy patients in cardiogenic shock, and have the unique ability to enable native heart recovery, allowing patients to return home with their own heart. The Impella RP® is U.S. FDA approved to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant or open-heart surgery. The Impella RP is also authorized for emergency use by healthcare providers (HCPs) in the hospital setting for providing temporary right ventricular support for up to 14 days in critical care patients with a body surface area ≥1.5 m2, for the treatment of acute right heart failure or decompensation caused by complications related to coronavirus disease 2019 (COVID-19), including pulmonary embolism (PE). The Impella RP has not been cleared or approved for the treatment of acute right heart failure or decompensation caused by complications related to COVID-19. Impella Left Ventricular (LV) Support Systems are also authorized for emergency use by HCPs in the hospital setting for providing temporary (≤ 4 days for Impella 2.5, Impella CP, and Impella CP with SmartAssist; and ≤ 14 days for Impella 5.0 and Impella 5.5 with SmartAssist) LV unloading and support to treat critical care patients with confirmed COVID-19 infection who are undergoing ECMO treatment and who develop pulmonary edema while on V-A ECMO support or late cardiac decompensation from myocarditis while on V-V ECMO support. The authorized Impella LV Support Systems have neither been cleared or approved for the authorized indication for use. The Impella RP and Impella LV Support Systems have been authorized for the above emergency use by FDA under an EUA and have been authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
In Europe, the Impella 2.5, Impella CP and Impella CP with SmartAssist are CE marked for treatment of high-risk PCI and AMI cardiogenic shock patients for up to 5 days. Impella 5.0 and Impella LD are CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 10 days. The Impella 5.5 with SmartAssist is CE marked to treat heart attack or cardiomyopathy patients in cardiogenic shock for up to 30 days. The Impella RP is CE marked to treat right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, open-heart surgery, or refractory ventricular arrhythmia. To learn more about the Impella platform of heart pumps, including their approved indications and important safety and risk information associated with the use of the devices, please visit www.impella.com.
ABOUT ABIOMED
Based in Danvers, Massachusetts, USA, Abiomed, Inc. is a leading provider of medical devices that provide circulatory support. Our products are designed to enable the heart to rest by improving blood flow and/or performing the pumping of the heart. For additional information, please visit: www.abiomed.com. Abiomed, Impella, Impella 2.5, Impella 5.0, Impella 5.5, Impella LD, Impella CP, Impella RP, SmartAssist and Impella Connect are registered trademarks of Abiomed, Inc., and are registered in the U.S. and certain foreign countries. Impella BTR, Impella ECP, CVAD Study and STEMI DTU Study are pending trademarks of Abiomed, Inc.
FORWARD-LOOKING STATEMENTS
This release contains forward-looking statements, including statements regarding development of Abiomed's existing and new products, the company's progress toward commercial growth, and future opportunities and expected regulatory approvals. The company's actual results may differ materially from those anticipated in these forward-looking statements based upon a number of factors, including uncertainties associated with the scope, scale and duration of the impact of the COVID-19 pandemic, development, testing and related regulatory approvals, including the potential for future losses, complex manufacturing, high quality requirements, dependence on limited sources of supply, competition, technological change, government regulation, litigation matters, future capital needs and uncertainty of additional financing, and other risks and challenges detailed in the company's filings with the Securities and Exchange Commission, including the most recently filed Annual Report on Form 10-K and the filings subsequently filed with or furnished to the SEC. Readers are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this release. The company undertakes no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201014005286/en/

