New government program enables access to integrated hybrid closed loop technology for
eligible residents
BRAMPTON, ON, Feb. 25, 2022 /CNW/ - Medtronic Canada ULC, a subsidiary of Medtronic plc (NYSE: MDT), a global leader in healthcare technology, applauds the Ontario government's announcement of a comprehensive reimbursement program for continuous glucose monitoring (CGM) for eligible residents living with type 1 diabetes (T1D). CGM devices provide critical information on glucose levels to help with the management of diabetes. Medtronic fully supports this investment in the health of Ontarians living with diabetes, enabling access to innovative diabetes management technology to help improve control of this very challenging chronic disease.
"This is a real game-changer," said Tori Lowe, 20. "Before I had to wake up several times a night to check my levels and now I won't have to. I already use a Medtronic pump, so now their CGM system can be integrated into my treatment plan. I can have more choice, more peace of mind, and I can order everything in one place. This will make a world of difference to help Canadians like me living with diabetes."
In Ontario, more than 300,000 people live with type 1 diabetes,1 and can spend between $1,000 to $5,000 each year out of pocket for their supplies, prescribed medication, and devices, averaging 3% of their annual income (without private insurance).2 These high costs remain the primary reason many patients do not adhere to their diabetes management plan, putting them at risk of developing serious, life-threatening conditions. Studies show that maintaining blood sugars in adequate range reduces long term health risks, 3,4 as well as improved glycemic outcomes in children and youth using hybrid closed loop technology.5,6
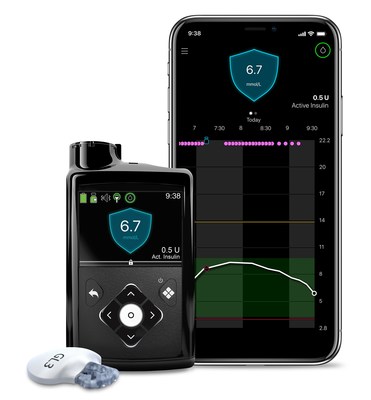
With the announcement of Ontario's public coverage program, eligible people living with type 1 diabetes can now access reimbursement for the integrated CGM — part of the Medtronic MiniMed™ 770G insulin pump system, allowing them to benefit from hybrid closed loop technology. This is currently the only system licensed by Health Canada offering adaptive insulin delivery for those as young as two years old. Patients can now also access the Medtronic Guardian Connect™ stand-alone CGM system, offering up to 60 minutes predictive alerts for low or high glucose levels.
"We recognize how important CGM is for people living with T1D, and since the day we brought the very first system to Canada — have been advocates for patient access to diabetes technology," says Laura Cameron, senior director of the Diabetes business at Medtronic Canada. "This will allow patients to choose the glucose measuring device that best meets their unique clinical and lifestyle needs, opening the door for even greater automated technologies in the future. We look forward to supporting patients with optimizing their diabetes management."
Details on eligibility requirements are available at: https://news.ontario.ca/en
For more information about accessing Medtronic CGM, visit www.medtronicdiabetes.ca/CGMaccess
About the Diabetes Group at Medtronic (www.medtronicdiabetes.ca)
Medtronic is working together with the global community to change the way people manage their diabetes. The company aims to transform diabetes care by expanding access, integrating care, and improving outcomes, so people living with diabetes can enjoy greater freedom and better health without compromising safety.
About Medtronic Canada ULC
Bold thinking. Bolder actions. We are Medtronic. Proud to serve Canadian healthcare for over 50 years, Medtronic Canada ULC is headquartered in Brampton, Ontario, with regional offices in Montreal and Vancouver, and is a subsidiary of Medtronic plc. We are the leading global healthcare technology company that boldly attacks the most challenging health problems facing humanity by searching out and finding solutions. Our Mission — to alleviate pain, restore health, and extend life — unites a global team of 90,000+ passionate people. Our technologies and therapies address 70 health conditions and include cardiac devices, surgical robotics, insulin pumps, surgical tools, patient monitoring systems, and more. Powered by our diverse knowledge, insatiable curiosity, and desire to help all those who need it, we deliver innovative technologies that transform the lives of two people every second, every hour, every day. Expect more from us as we empower insight-driven care, experiences that put people first, and better outcomes for our world. In everything we do, we are engineering the extraordinary. For more information on Medtronic Canada, visit www.Medtronic.ca and follow @MedtronicCA on Twitter and LinkedIn.
Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results.
- Data on file; Arcus Consulting Group
- Diabetes Canada. Diabetes in Ontario – Backgrounder. 2021.
- Vigersky RA. Diab Tech Ther.2019; 21:81-85
- Beck R et al. Diabetes Care. 2018 Oct; epub ahead of print dc181444. 3. Beck RW, et al. JAMA. 2017;317(4):371-378
- Forlenza, GP et al. Diab Tech Ther. 2019; 21(1), 11–19.
- Forlenza GP, et al. Pediatr Diabetes. 2022 Jan 10. doi: 10.1111/pedi.13312. Epub ahead of print.
SOURCE Medtronic Canada ULC
© Canada Newswire, source Canada Newswire English





